standardize sodium thiosulfate
Standardize a sodium thiosulfate Na2S2O3 solution for a titration experiment. Sodium thiosulfate sodium thiosulphate is an inorganic compound with the formula Na 2 S 2 O 3.

0 1 N Sodium Thiosulfate Preparation And Standardization
211 Vitex indicator laboratory grade 3.

. Starch indicator is typically used. The student adds a 100 mL aliquot of 00200 M KIO3 solution to a flask followed by 3 mL of distilled water 02 g of solid KI and 1 mL H2SO4 Question. The iodine liberation process significantly affects the titration results.
SODIUM THIOSULFATE CAN BE STANDARDIZED AGAINST PURE IODINE DISSOLVED IN KI OR AGAINST IODINE SET FREE FROM AN ACIDIFIED SOLUTION OF KI BY STANDARD SOLUTION OF POTASSIUM PERMANGANATE OR VARIOUS PRIMARY STANDARD OXIDIZING AGENTS SUCH AS POTASSIUM IODATE. Download thiosulfate standardization against iodine reaction file open it with the free trial version of the stoichiometry calculator. Dissolve 6205 g Na2S2O35H2O in reagent grade water.
KI I2 KI3. To 500 ml of this solution add 2 g of potassium iodide and 3 ml of 2 M hydrochloric acid and titrate with the sodium thiosulphate solution using the starch solution added towards the end of the titration as an indicator until. Actually the iodine solution 01 N or 1 N is prepared by mixing potassium iodide to the iodine obtaining the complex KI3.
In aqueous solution sodium thiosulfate dissociate into ions. This method is developed using an automated titration system Excellence Titrator. Potassium iodate a strong oxidizing agent is treated with excess potassium iodide in acidic media which liberates iodine which is back titrated with sodium thioslphate.
Hello I need to prepare a 01N solution of sodium thiosulfate and standardize with potassium iodate in the guide it uses 3 ml of sulfuric acid but I have hydrochloric acid and I need help to determine how much I should use. S2O3- aq Haq S s SO2 g H2O l. ASTM D1510-16 also describes the standardization method for sodium thiosulfate solution using KIO 3 KI as a primary standard.
I have two questions here. Iodine is the standard. The solution is shaken again then covered and keept in a dark place.
What primary standard can be used to standardize sodium thiosulphate. It is used as a titrant in iodometry. In this process first we need to liberate iodine to react with sodium thiosulfate.
The first section is to standardize the Sodium Hydroxide by titration. The pentahydrate of this salt has a melting point of 3214 K and a boiling point of 373 K. Take 100 mL distilled water in conical flask Add 2g of Potassium di-iodide Add 10 mL of potassium di chromate And lastly add 2.
X H 2 O. 50 ml of distilled water are added to each three of it from graduated cylinder and constantly shake it until the KHP solution are completely Premium Sodium hydroxide Titration. Sodium Thiosulfate solutions are almost exclusively used to standardize Iodine solutions or as back-titrants in titrations using Iodine.
NaS2O3 Na aq S2O3- aq as the acid is titrated into the sodium thiosulfate solution the acidity of solution increases. Uniformity of reactions between iodine and sodium thiosulphate forms. 9778BNWP or 9678BNWP and a standard solution of sodium thiosulfate.
To do so he or she will react it with a solution of iodine. Introduction Sample Preparation and Procedures Chemistry Instruments and Accessories Method in Detail. To standardise 0025 N sodium thiosulfate.
First sodium bicarbonate is added to a iodate-free solution of potassium iodide. Typically it is available as the white or colorless pentahydrate Na 2 S 2 O 3 5H 2 OThe solid is an efflorescent loses water readily crystalline substance that dissolves well in water. In the standardization iodine triiodide liberated by potassium dichromate in an acidic potassium iodide solution is titrated with a sodium thiosulfate solution.
Sodium thiosulfate is often standardized with potassium dichromate. Procedure Dissolve approximately 2 g KI in an Erlenmeyer flask with 100 to 150 mL DI water. I apologize for my English and thank you very much.
The mixture is shaken until the salts dissolve then conc. Sulphite E225 sodium metabisulfite E223 potassium metabisulfite E224 all used as preservatives. Sodium thiosulfate is used in gold mining water treatment analytical chemistry the.
Add 2 drops of CHCl3. Sodium potassium thiosulfate. The solubility of sodium thiosulfate in water is 701g100 mL at 20 o C and 231g100mL at 100 o The crystal structure of Na 2 S 2 O 3 crystals is monoclinic.
Standardization of 01 M Sodium thiosulfate solution Dissolve 0200 g of potassium bromate weighed accurately in sufficient water to produce 2500 ml. Sodium thiosulfate 001N standardized solution is used in volumetric analysis to estimate the concentration of certain compounds in solution hydrogen peroxide as well as chlorine content in bleaching powder and water. The principle of standardization of sodium thiosulphate is based on redox iodometric titration with potassium iodate as primary standard.
Three sample of 07 09 g of solid KHP are place into each of the three numbered Erlenmeyer flasks. Typically it is best to standardize daily or weekly. Introduction Since the concentration of the iodine titrant changes over time for best accuracy determine the true concentration of the iodine titrant by standardizing with a standard solution of sodium thiosulfate.
Click nCV button over iodine. Hydrochloric acid is added. Add 15 mL 6 N NaOH or 04 g solid NaOH and dilute to 1 L.
Leaving Cert Chemistry- By kind permission of Folens. To calculate thiosulfate solution concentration use EBAS - stoichiometry calculator. Produce sulfur see CLEAPSS.
210 Standard sodium thiosulphate solution 0025 N. For a 15-minute exposure concentration of metabisulfite in the. Student Safety Sheet 82 sulfur dioxide TOXIC.
Solutions of Sodium Thiosulfate are most commonly standardized with Potassium Dichromate or Potassium Iodate solutions which generate Iodine from Iodide. In acidic condition abundance of H ion thiosulfate ion is unstable. The density of sodium thiosulfate corresponds to 1667 grams per cubic centimetres.
Enter concentration and volume of the sample click Use button.

0 1 N Sodium Thiosulfate Preparation And Standardization

How To Prepare Standardization 0 1 N Potassium Permanganate Colored Glass Bottles Glass Containers Potassium

Preparation And Standardization Of Sodium Thiosulfate By Generose Carumba
Standardize Sodium Thiosulfate According To Astm D1510 16

How To Prepare Standardization 0 1 N Potassium Dichromate

Standard Preparation Chemistry Experiments Life Science Chemical Reactions

0 05 M Edta Solution Preparation Standardization
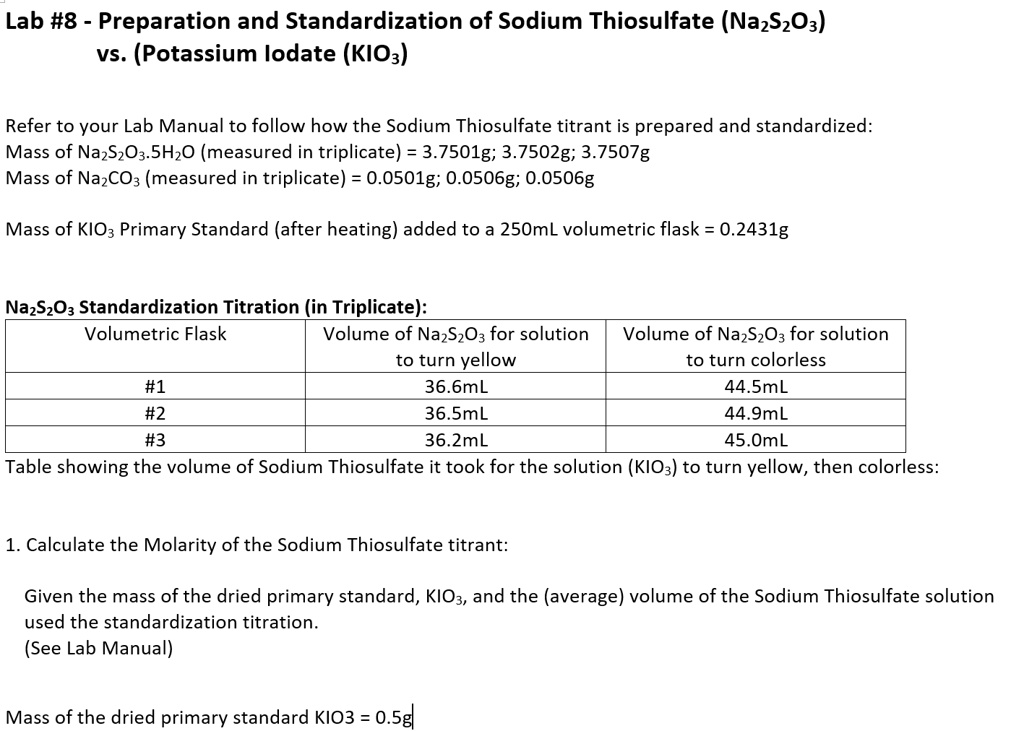
Lab 8 Preparation And Standardization Of Sodium Thios Itprospt
Sodium Thiosulfate 0 1n Standardized Solution Thermo Scientific

Standardisation Of Sodium Thiosulphate 0 02 Molar Solution Of Sodium Thiosulphate Youtube

How To Prepare Standardization 0 1 N Sodium Hydroxide Naoh Sodium Hydroxide Sodium Preparation

Standardization Of Sodium Thiosulphate Solution With Standard

Chrominfo Preparation And Standardization Of 0 1 N Sodium Thiosulphate

0 005 M Sodium Thiosulfate Standard Solution Dilute 50 Cm3
0 Response to "standardize sodium thiosulfate"
Post a Comment